Cell Culture Basics: Equipment, Fundamentals and Protocols

New to cell culture? Then look no further. Here you will find a basic overview of all things cell culture, from setting up a cell culture lab to understanding basic principles and fundamental techniques. A great starting point to springboard into the world of cellular biology.
Basic equipment and reagents required for cell culture
Cell culture laboratory design
Cell culture safety
Cell culture basics
- Culture conditions
- Primary vs immortalized cells
- Adherent vs suspension cultures
- Mammalian vs non-mammalian culture
- Cell growth and confluency
- Choosing a cell line
- Cell line authentication
- Cell culture media: picking the right media for your cells
Standard cell culture protocols
- Aseptic technique
- Primary cell isolation
- Subculturing/passaging cells
- Cryopreservation and thawing
- Testing cells for mycoplasma infection
- Cell counting
- Cell transfection
What is cell culture?
Cell culture refers to the removal of cells from an animal or plant and subsequent cultivation in an artificial environment for scientific research. The first cell culture techniques were developed over 100 years ago and since then have contributed to tremendous breakthroughs in science. Today, it is afundamental toolused in laboratories around the world for studying the normal physiology and biochemistry of cells,mechanisms underlying disease, includingcancer,and effects ofdrugsand toxic compounds. It is also used indrug screening and development生物compou和大规模生产nds, such as vaccines andtherapeutic proteins.1,2There's also a growing role of cell culture in the food industry both fortesting for contaminantsand incellular agricultureandcultured meatproduction to ease environmental burdens.
Basic equipment and reagents required for cell culture
To conduct research requiring cell culture work and to perform fundamental cell culture protocols, there are several key pieces of equipment and some basic reagents that are required, summarized in Tables 1 and 2.
Table 1:Basic equipment required for cell culture.3,4,5The images were created with BioRender.com.

Table 2:The basic reagents needed for cell culture.2,5
Complete medium
|
See section below on media for your cells. |
|
Buffered solution
|
Phosphate-buffered saline (PBS) used for washing cells. |
|
Detaching agent
|
An enzyme used to detach adherent cells from culture vessels for culturing, such as trypsin. |
|
Cryoprotective agent |
An agent thatreduces the freezing point of media and slows the cooling rate to reduce the risk of ice crystal formation which can damage cells and cause cell death. Dimethylsulfoxide (DMSO) is most commonly used. |
It is also important to have access to deionized and distilled water as well as ice. The nature of the experiments to be performed will inform the reagents that will need to be acquired.
Cell culture laboratory design
有几个重要的设计考虑required for any cell culture setup. The most important aspect is using design to maintain an aseptic and sterile environment toprevent the contamination of cells.首先,一个独立的封闭的房间或实验室with one entry/exit point should be used. Hand wash sinks with soap and sanitizer should be close by for hand cleaning on entry and exit of the lab. Dedicated cell culture lab coats and safety goggles should be stored at the lab entrance. The laminar flow hood and incubators should be away from the entrance to minimize contamination risk. It is also important to position the hood and incubator away from any air conditioning units to prevent potentially contaminated airflow from entering the sterile work environment and incubators. There should be ample clear work surfaces that need to be sterilized regularly with sufficient storage space to ensure the surfaces remain clear. All necessary equipment and consumables should be accessible within the laboratory to prevent exiting and re-entering. An ergonomic environment is important for the laminar flow hood with sufficient room for drawers or moveable trolleys of consumables to be at hand when working as well as easy access to the incubator, microscope andcentrifuge.6It's also important to use appropriate plastic consumables thatminimize the risk of extractable compounds leaching outand contaminating your cell cultures.
Cell culture safety
A cell culture laboratory poses risks associated with handling and manipulating cells and tissues as well as toxic, corrosive or mutagenic solvents and reagents. Therefore, adherence to standard microbiological practices and techniques is of paramount importance to mitigate risks and ensure safety at all times. There are four ascending levels of biosafety containment, referred to asbiosafety levels(BSL). Each level has standard microbiological practices, safety equipment and facility safeguards to be implemented when dealing with hazardous biomaterials and agents. BSL-1 is the basic level of protection common to most research and clinical laboratories where the agents used are not known to cause disease in normal, healthy humans. BSL-2 is appropriate for moderate-risk agents known to cause human disease of varying severity by ingestion or through percutaneous or mucous membrane exposure. Most cell culture labs should be at least BSL-2, but the exact requirements depend upon the biomaterials used and the type of work conducted. BSL-3 is required for agents that pose a serious and potentially lethal infection and BSL-4, the highest containment level, is required for laboratories working with infectious agents that pose a high individual risk of life-threatening disease.4,7
The following is a list of basic safety recommendations for a cell culture laboratory. The list is by no means complete and should be supplemented with the appropriate biosafety level recommendations.
- Always wear appropriate personal protective equipment (PPE) including laboratory coat, gloves and safety goggles.
- Always read the material safety data sheet (MSDS) for any substance you are working with to ensure appropriate safety precautions when handling.
- Decontaminate all work surfaces before and after your experiments.
- Clean laboratory equipment routinely, even if it is not contaminated.
- Avoid the creation of aerosols and/or splashes.
- Wash your hands after working with potentially hazardous materials and before leaving the laboratory.
- Decontaminate all potentially infectious materials before disposal.
- Report any incidents that may result in exposure to infectious materials to appropriate personnel (e.g., laboratory supervisor, safety officer).
- Do not eat, drink, smoke, handle contact lenses, apply cosmetics, or store food for human consumption in the laboratory.
Cell culture basics
Culture conditions
For cell survival and proliferation, it is essential that the culture environment replicates, as best as possible, the physiological environment of cells. Culture conditions that can be controlled include temperature, relative humidity and CO2levels as well as factors associated with the media, such as nutrient composition, pH, osmolality and the volume and frequency of replenishment. These variables can fluctuate over time so they should bemonitored.Table 3 highlights the optimal culture conditions for most mammalian cell cultures, however exceptions do exist.5
Table 3:Optimal cell culture conditions for most mammalian cells.1,2,4,8
pH
|
7.0–7.4
|
|
Osmolarity
|
280–320 mOsmol/kg |
|
CO2
|
5–10%
|
|
Temperature |
35–37 ◦C
|
Primary vs immortalized cells
Primary cell cultures are cells isolated directly from intact or dissociated tissues or from organ fragments and grown in a dish. Once a primary culture has been sub-cultured for the first time it becomes known as a cell line. Primary cell lines have a finite lifespan and can only be sub-cultured 10–20 times before reaching a state of senescence (cessation of cell division).1
Some cell lines have no limit on their lifespan and have an infinite capacity to proliferate. These cell lines are known as continuous orimmortalized cell lines.Immortalization of cells can occur in numerous ways. Cancerous cells have inherent mutations that enable the cells to propagate without limits in culture. Normal cells that initially have a limited lifespan can transform to become immortalized through mutations in growth promoting genes. Normal cells grown in culture can also be intentionally immortalized by treatment with chemicals or introduction of a tumor-causing virus that activates growth-promoting genes. Figure 1 shows the evolution of primary to transformed continuous cell lines and the theoretical cell yield from each phase.9
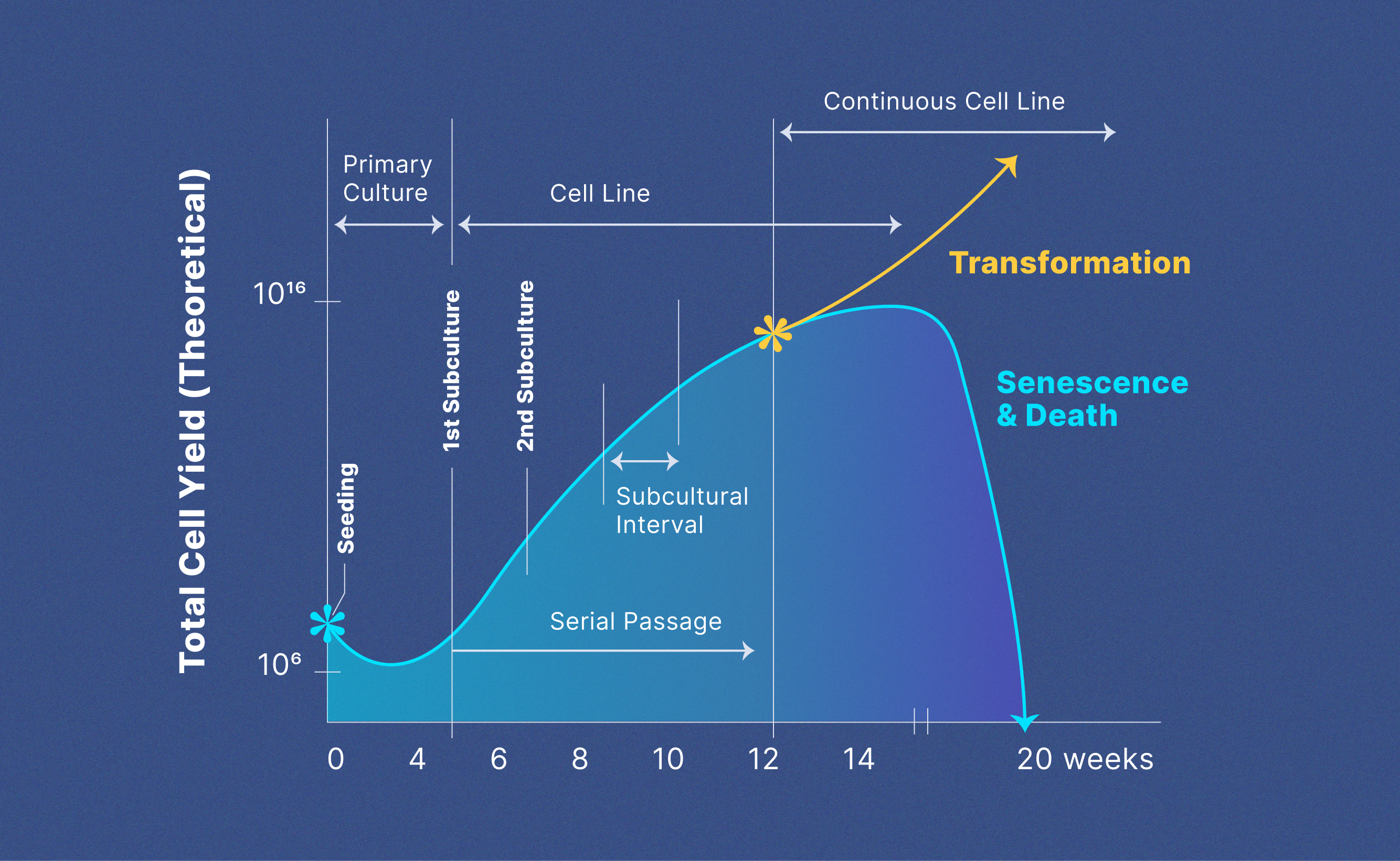
Figure 1: The evolution of primary to continuous cell lines and theoretical cell yield.
Adherent vs suspension cultures
Adherent cells grow in a monolayer attached to the cell culture vessel surface. When passaged, a detaching agent needs to be used to detach them from the surface. They re-attach to the surface within a few hours upon plating. Suspension cells do not form monolayers on the surface of cell culture vessels, but remain in suspension. Cells form clumps, especially at high density.1,10
Mammalian vs non-mammalian culture
Mammalian cell cultures are most common, however, cells from a plethora of organisms can be cultured, such as plants, insects, bacteria and yeast. Plant cell cultures are typically grown as cell suspension cultures in a liquid medium or as callus cultures on a solid medium. Cells derived fromDrosophila melanogasteror armywormSpodoptera frugiperdaare examples of insect cell lines that are used for biochemical assays or expression of recombinant proteins respectively. For bacteria and yeast, small quantities of cells are usually grown on a solid support that contains nutrients embedded in it, usually a gel such as agar, while large-scale cultures are grown with the cells suspended in a nutrient broth.
Cell growth and confluency
Cell culture growth generally occurs in four phases (Figure 2). Lag phase occurs when cells are acclimatizing to culture conditions and are not dividing. Log phase occurs when cells are actively dividing. This is the best phase for cell experimentation and data collection. Cells should be sub-cultured when they reach late log phase. This occurs just before overcrowding. When cells approach overcrowding, cell growth slows. This is known as stationary phase or plateau phase. Cells in this phase are at risk of cellular stress. When the natural process of cell death predominates, a cell population is considered to be in death phase, also referred to as decline phase. When the log of the cell count over time is graphed, it generates a sigmoidal curve as depicted in Figure 2. It is important to note that the amount of time spent in each phase differs between individual cell lines and cultures.11

Figure 2: The four phases of cell growth.
An important metric to describe monolayer cell culture is confluence. It is the percentage of the culture vessel surface area that appears covered by a layer of cells when observed by microscopy. For example, when half of the surface area of a culture vessel is covered by cells, the population is considered to be at 50% confluence. The schematic in Figure 3 shows examples of different cell confluences. Cell density is used to describe cells that are grown in suspension.2,5
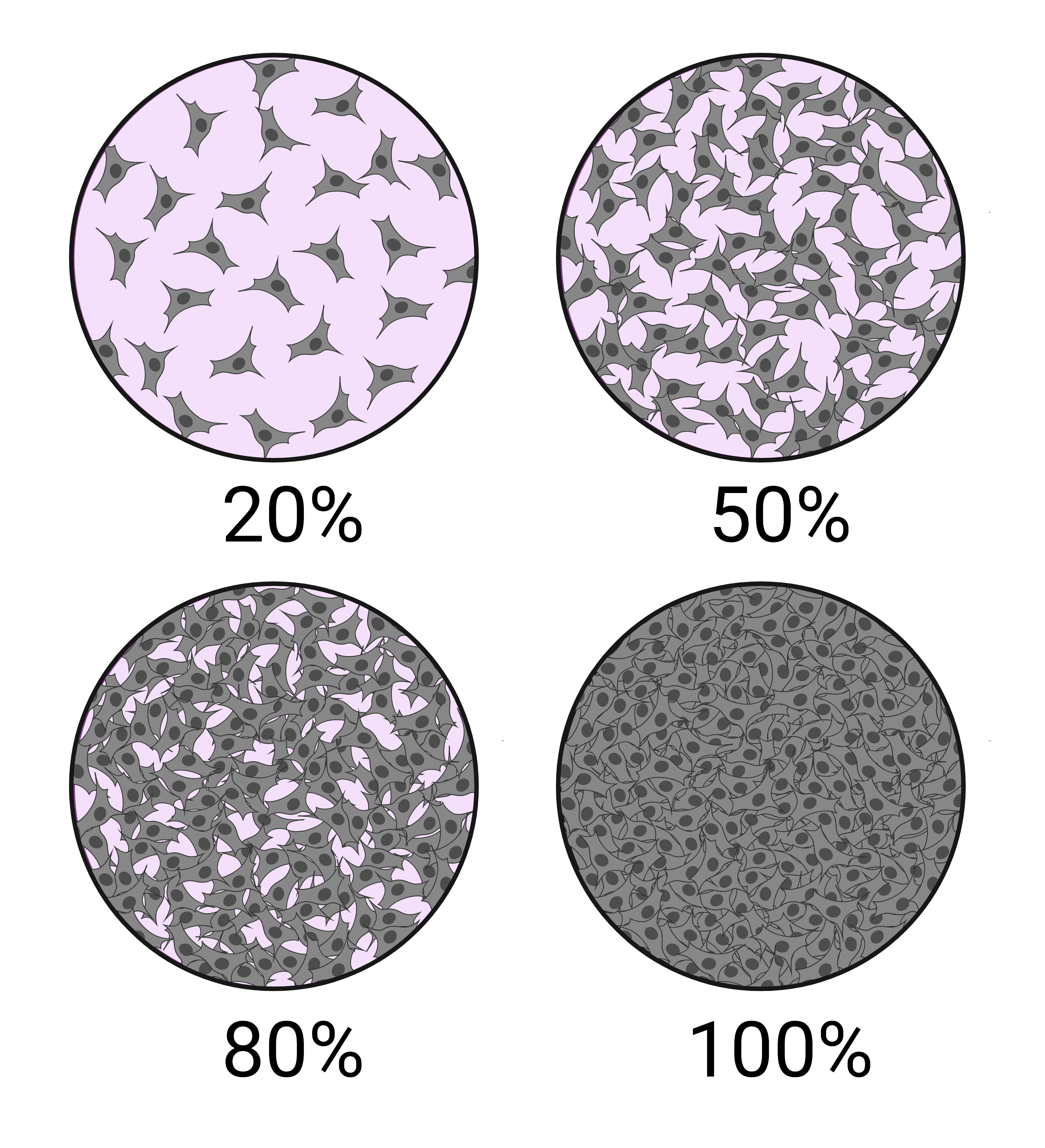
Figure 3: A schematic showing 4 fields of view under a microscope of cells depicted at 20%, 50%, 80% and 100% confluence. This image was created with BioRender.com.
Choosing a cell line
There are thousands of established cells lines used in laboratories around the world that can be purchased from commercial or non-profit suppliers (cell banks). It is critical to obtain cell lines from reputable suppliers as cells are verified and contamination-free. Obtaining cell lines from other laboratories has a high risk of contamination and lack of cell line validation and is therefore advised against.
The criteria in Table 4 should be taken into consideration whenselecting the appropriate cell linefor an experiment. The cell line chosen will largely depend on the nature and requirements of the experiment to be performed.
Table 4:Criteria to consider when selecting a cell line.1,2
Species
|
Does a species-specific cell line need to be used? If not, non-human and non-primate cell lines usually require reduced biosafety restrictions which may be favorable. |
|
Functional characteristics
|
Use an appropriate cell line for your experiment. For example, liver- and kidney-derived cell lines may be more suitable for toxicity testing. |
|
Finite or immortalized
|
Finite cell lines are more functionally relevant as they have not undergone immortalization, however immortalized cell lines are often easier to maintain and clone. |
|
Normal or transformed |
Transformed cell lines have an increased growth rate and higher plating efficiency which is favorable, but the cells have undergone a permanent genetic change. Does this potentially impact your experiment? |
|
Growth conditions and characteristics |
Is growth rate, cloning efficiency, or saturation density important for your experiment? Do you need cells to be adherent or in suspension? For example, if you want to express a recombinant protein in high yields you would choose a fast-growing cell line that can grow in suspension. |
Table 5 lists 20 commonly used cell lines and their characteristics including species, tissue origin and morphology. The morphology of cells is generally described as fibroblast, epithelial or lymphoblast which indicates both cell of origin and physical appearance. Fibroblast cells are bipolar or multipolar and elongated in shape; epithelial cells have a polygonal shape and in general show more regular dimensions and lymphoblast cells show a spherical outline and are typically grown in suspension.1,5
Table 5:20 commonly used cell lines and their characteristics.
Cell line |
Origin |
Species |
Morphology |
Culture type |
Cervix |
Human |
Epithelial |
Adherent |
|
Embryonic kidney transformed with adenovirus |
Human |
Epithelial |
Adherent |
|
Peripheral blood |
Human |
Lymphoblast |
Suspension |
|
Human promyelocytic leukemia cells |
Human |
Lymphoblast-like |
Suspension |
|
Breast cancer |
Human |
Epithelial-like |
Adherent |
|
Bone |
Human |
Epithelial |
Adherent |
|
Prostate |
Human |
Epithelial |
Adherent, can be adapted to suspension |
|
Lung cancer |
Human |
Epithelial-like |
Adherent |
|
Liver cancer |
Human |
Epithelial-like |
Adherent |
|
Bone marrow |
Human |
Epithelial |
Adherent or suspension |
|
Embryonic stem cells |
Human |
Lymphoblast |
Suspension |
|
Ovary Chinese |
Hamster |
Epithelial |
Adherent |
|
Kidney epithelial cell |
African green monkey |
Epithelial |
Adherent |
|
Kidney cells |
African green monkey |
Fibroblast |
Adherent |
|
Fibroblast |
Mouse |
Fibroblast |
Adherent |
|
Fibroblast |
Syrian hamster |
Fibroblast |
Adherent |
|
Epithelial cell |
Dog |
Epithelial |
Adherent |
|
Myoblast |
老鼠 |
Myoblast |
Adherent |
|
Embryonic fibroblast cells |
Zebrafish |
Fibroblast |
Adherent |
|
Ovaries |
Fall armyworm (Spodoptera frugiperda) |
Epithelial |
Suspension or adherent |
Cell line authentication
Cell culture is an important tool in laboratories around the world. However, cell lines can be misidentified or contaminated with other cells. This invalidates published data and wastes laboratory time and resources. Due to the magnitude of this issue and the need to ensure valid and reproducible results, journals, institutions, and funding bodies now recommend or requirecell line authentication.Cell line authentication can be achieved by genetic profiling using polymorphic short tandem repeat (STR) loci. Cells should be authenticated upon receipt of a new cell line and at regular intervals during sub-culturing.12
Cell culture media: picking the right media for your cells
Cultured cells are fed withliquid media.There are four key ingredients to consider when making up medium for your cells which are detailed in Table 6. The specific details of the medium requirements for your cells can be found on the data sheet. Always make sure to check the data sheet details on culturing conditions when culturing a new cell line.
Table 6:Key components of mammalian cell culture media.1,13,14
Basal medium
|
Basal medium is a mixture of nutrients and salts. There are various formulations which includeminimum essential medium (MEM), Dulbecco's modified Eagle’s medium (DMEM) and Roswell Park Memorial Institute (RPMI). They can be purchased in liquid form or powder form from commercial sources. |
|
Glutamine
|
Glutamine is an essential amino acid that is necessary for cell growth. Basal medium may be purchased with glutamine, without glutamine, or with a stable dipeptide glutamine substitute. It is important to make sure that your cultures have glutamine under normal growth conditions. |
|
Animal serum |
Cells are often grown in basal medium supplemented with animal serum. This becomes known as “complete” medium. Serum provides required growth factors and nutrients for cells. Fetal calf (fetal bovine) serum is most commonly used. In most cases, serum is added so that the final volume in the complete medium mixture is between 5% and 20%. |
|
Antibiotics |
Cell culture medium is often supplemented with an antibiotic combination of penicillin and streptomycin as a measure to prevent bacterial growth. With aseptic technique, it is possible to grow healthy cell cultures without the use of antibiotics. |
Standard cell culture protocols
There are several fundamental cell culture protocols that are performed in all cell culture laboratories. It is important to become familiar with and understand these protocols.
Aseptic technique
Aseptic technique, when practiced consistently, can help to ensure the sterility of all culture media and culture vessels, thereby reducing the chance of cells being exposed tocontaminantsand keeping cultures healthy, viable, and pure. Fastidious aseptic technique is an essential prerequisite for successful cell culture to keep cultures free from bothmicrobial contaminationandcellular cross contamination.Aseptic technique covers handling, reagents and workplace which is summarized in Table 7.15,16
Table 7:A summary of aseptic techniques required when working with cell culture.
Handling |
Reagents/media
|
Workplace
|
·Gentle and careful handling.
·Sterilization of all items before starting.
·Sterile pipettes, pipette tips and plasticware.
·No touching of sterile items to non-sterilized surfaces (including gloved hands to your own skin, clothes or hair). |
·Pre-sterilization of all reagents and equipment.
·No contamination visible in reagents.
·Aliquoting reagents into smaller volumes to work from prevents contamination of an entire stock.
·Having your own working stocks removes the risk of contamination from shared stocks. |
·Check the culture hood works properly.
·Work area always sterile and tidy.
·Frequent cleaning and de-contamination of hood, incubators and fridges. |
Primary cell isolation
Primary cell isolation can be performed on a variety of complex biological samples including tissues (skin, liver, tumor, brain, lung etc.), bone marrow, blood, spleen and lymph nodes. There are many different ways to prepare samples for optimal cell isolation. The method you select depends on your starting sample and may involve removing certain elements from it or simply creating a single-cell suspension. When isolating cells from intact tissues, you must first disrupt the extracellular matrix holding the cells together using mechanical force and/or proteolytic enzymes (Table 8). 17
The basic outline of primary cell isolation requires the isolated piece of tissue to be minced or cut into 2–4 mm pieces with sterile scissors or a scalpel. The tissue pieces are added to an appropriate buffer or balanced salt solution on ice and washed 2-3 times. Dissociation enzymes are added according to the protocol and incubated. Cells are dispersed by gently pipetting. The cell suspension is filtered through a fine mesh and washed 2-3 times. Cells are resuspended in medium and seeded.18
Table 8:Enzymes commonly used in tissue dissociation protocols for cell isolation.18
Collagenase
|
Hydrolyzes collagen and is widely used for isolating cells from animal tissues. |
|
Hyaluronidase
|
胶原酶和催化结合使用hydrolysis of 1,4-β-D-glycosidic linkages. |
|
DNase
|
Added to cell suspensions to minimize cell clumping due to DNA released by damaged cells. |
|
Elastase
|
Used to digest tissues containing high amounts of elastin. |
|
Trypsin
|
A serine protease with a specificity for peptide bonds and is often combined with other enzymes (e.g., elastase and/or collagenase) for tissue dissociation. |
Subculturing/passaging cells
Subculturing or passaging refers to the diluting of cells that have reached high confluence to enable continuous culture propagation. Adherent cells should be passaged when they are 80–90% confluent and suspension cultures should be passaged when cells start to clump and the culture becomes cloudy. It is important to keep a record of the number of cell passages for each cell line. This helps to monitor the viability and plan experiments for primary cells before they reach senescence. It helps to monitor the age of immortalized cells as the higher the passage number, the further the genetic drift. Experiments should not be performed on cell lines with very high passage numbers . 4
It is important to work gently with cells at all times as vigorous or harsh handling can result in cell damage or death. Never pipette medium or wash buffer directly onto cells, always add it gently to the side of the vessel to avoid harming the cells. When resuspending a pellet or triturating to mix cells, do so gently.
简单地说,附着ce的接种协议lls is as follows: media is removed and cells are washed once with PBS. A detaching agent is added, such as trypsin (breaks down the proteins that enable the cells to adhere to the vessel) and the cells are incubated at 37 °C until they are fully detached. Detachment can take anywhere from 1–20 minutes depending on the cell line. Monitor cells under a microscope to determine when detachment occurs. Inactivate the trypsin by adding complete medium to cells (the serum inactivates trypsin as it contains protease inhibitors) and spin in acentrifuge(3-5 minutes at 150–300 x g) to pellet cells. Remove media (liquid on top of the pellet), gently resuspend cells in fresh media and plate cells in a new culture vessel at the desired density. For suspension cells, no trypsin is required. Cells are collected and centrifuged (3-5 minutes at 150–300 x g) to form a pellet, media is removed and cells are resuspended in PBS for a wash step. The buffer is removed after another round of centrifugation and cells are resuspended in fresh media and replated at the desired density.5
Cryopreservation and thawing
Cell lines are valuable resources, so it is vitally important that stocks are preserved for long-term storage.Cryopreservationrefers to the process of cooling and storing cells at very low temperatures to maintain their viability. Cells are suitable for long-term storage at temperatures below -130 °C.
The best method forcryopreserving cultured cells地中海是储存在液氮完成吗ium in the presence of a cryoprotective agent such as DMSO.Cryoprotective agentsreduce the freezing point of the medium and also allow a slower cooling rate, greatly reducing the risk of ice crystal formation which can damage cells and cause cell death. Cells should be cryopreserved at a high concentration (e.g., 90% confluent) and at the earliest passage number possible. Briefly, cryopreservation includes washing and pelleting cells, resuspending them in complete medium with DMSO (e.g., 5% DMSO) and transferring the cell suspension solution to 1 mL sterile cryovials. The cryovials are placed in a controlled rate cryo-freezer or a cryo-freezing container as cells must be frozen slowly. The cryo-freezing container is stored at -50 to -80 °C for 24 hours and then the cryovials are transferred to liquid nitrogen storage.2,8
The exact freezing conditions depend on the cell line in use. It is very important to check cell line-specific conditions otherwise your frozen stocks may not produce viable cells when they are thawed and re-cultured.
Torevive cell linesfrom liquid nitrogen storage, frozen vials are transported in a portable liquid nitrogen container or dry ice to the cell culture area. The DMSO in the cryovials is toxic to cells once they are thawed, so to ensure high cell viability, cells must be thawed rapidly in a 37 °C water bath and immediately transferred to a culture dish with pre-warmed media. The media dilutes the DMSO so it is no longer at a toxic concentration. Once thawed cells have propagated and been passaged twice, they can be used for experimentation. It is good practice to freeze down more cells as soon as possible and replace the vial that was removed from long-term storage.2
Testing cells for mycoplasma infection
A major problem in cell culture ismycoplasma infection.This bacterial infection can alter cell behavior and metabolism and have adverse effects on cells. It is very important to perform mycoplasma detection assays on a regular basis, especially for a continuous cell line. Good practice is to test for mycoplasma when a new cell line is received, a stock is thawed and cultured, just before freezing down and every 4–6 weeks for cell stocks in culture. An easy and reliable method for detecting mycoplasma is to stain a sample of cells with Hoechst 33258, a fluorescent dye that binds specifically to DNA, and view under a fluorescence microscope. Mycoplasma-free cells show clear and clean nuclear Hoechst staining, while mycoplasma-infected cells also show patterns of filamentous staining outside of the nuclei which is the bacterial DNA (Figure 4).5,19,20
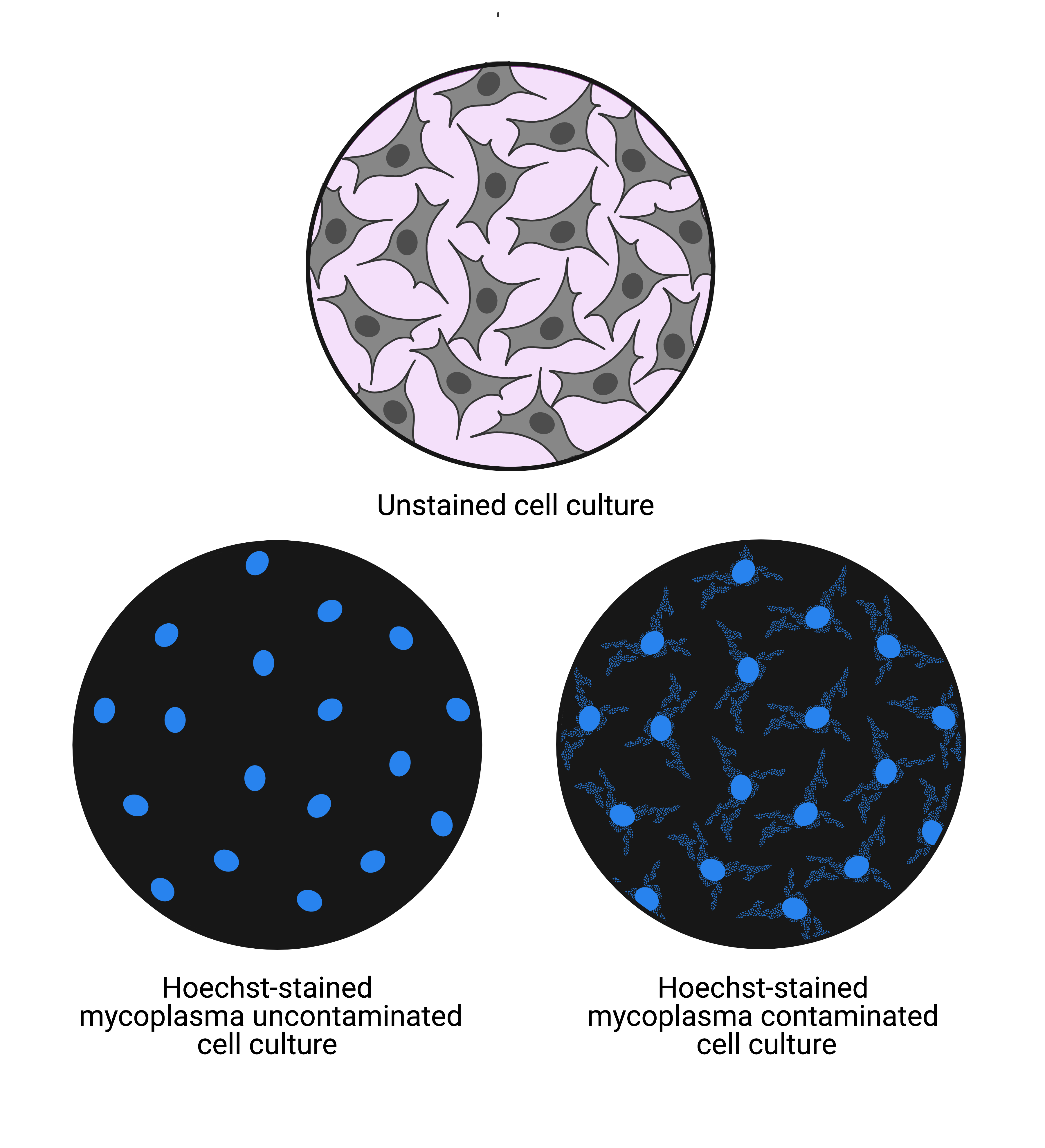
Figure 4: 无污点的示意图显示字段的视图(top) cells and cells stained with Hoechst 33258 that are mycoplasma uncontaminated (bottom left) and mycoplasma contaminated (bottom right). Uncontaminated cell culture shows nuclei staining while contaminated cell culture shows nuclei staining and cytoplasmic staining. The cytoplasmic staining indicates the presence of mycoplasma bacterial DNA. 5 This image was created with BioRender.com.
Cell counting
Despite the recent development of automated cell counters, manual cell counting using a hemocytometer is still the most commonly used method. A hemocytometer consists of a thick, glass microscope slide with two grids of etched perpendicular lines forming chambers. A thin cover glass is provided too. Always clean the hemocytometer and cover glass before use and position the cover glass over the chambers. Add 10–20 μl of cell suspension into one of the two chambers on the microscope slide under the cover glass. Use an inverted phase contrast microscope at 20 x magnification to count the cells in each of the four outer squares as depicted in Figure 5. You should aim to be counting 100–200 cells per square for accurate results. If you have too few cells, then resuspend your cells in less media next time. If you have too many cells to count, then resuspend your cells in a higher volume of media. Once you have counted each corner, add the counts together and divide by four. Your cell concentration will be your count x 104cells/mL. To work out total cells, multiply the concentration by the cell suspension volume. For example, 5 mL of cell suspension with a concentration of 80 x 104cell/mL is a total of 400 x 104cells or 4 x 106cells or 4 million cells.4,5,21,22

Figure 5: Counting cells with a hemocytometer. The top panel shows an aerial and side view schematic diagram of a hemocytometer. The bottom left panel shows what the counting chamber looks like under a microscope. The red boxes highlight the quadrants to be counted. The bottom right panel is an example of how to count cells within a quadrant.
Cell transfection
Transfection of cellsrefers to the delivery of nucleic acid (DNA or RNA) into cultured cells. The most commonly used reagents are cationic lipids that can associate with nucleic acids to form positively charged complexes and allow interaction of DNA/RNA with the negatively charged cell membrane. This leads to the efficient entry of nucleic acids into cells via endocytosis. This is often called lipofection or lipid transfection. Alternatively, nucleic acids can be delivered into cells by electroporation, co-precipitation of DNA with calcium phosphate, or polybrene/DMSO shock. The delivered DNA does not usually integrate within the host genome and is therefore transient. To achieve stable expression or knock down of a gene, stable cell lines can be engineered. This requires DNA vector design and antibiotic selection of cells where DNA has stably integrated into the genome.23,24
A lipofection transfection protocol is very straightforward. In essence, a solution containing the right concentration of DNA/RNA for transfection is prepared and a solution containing the lipofection reagent is prepared. The two solutions are mixed together and incubated for a period of time depending on the protocol. The solution is then added to 60–80% confluent cells and experiments can be performed as early as 6 hours after transfection. The transient transfection generally lasts up to 72 hours.25
References
1. Verma A. Animal Tissue Culture: Principles and Applications.Animal Biotechnology: Models in Discovery and Translation.Elsevier Inc.; 2013:211-231.doi: 10.1016 / b978 - 0 - 12 - 416002 - 6.00012 - 2
2. Davis, John M, ed.Animal Cell Culture: Essential Methods.John Wiley & Sons Ltd; 2011.https://www.wiley.com/en-us/Animal+Cell+Culture%3A+Essential+Methods-p-9780470666586
3. Clarke S, Dillon J. The Cell Culture Laboratory.Animal Cell Culture: Essential Methods.John Wiley and Sons; 2011:1-31.doi:10.1002/9780470669815.ch1
4. Sandell L, Sakai D. Mammalian Cell Culture.Curr Protoc Essent Lab Tech.2011;5(1):4.3.1-4.3.32.doi:10.1002/9780470089941.et0403s5
5. ATCC. Animal Cell Culture Guide. Published 2021.https://www.atcc.org/~/media/PDFs/Culture Guides/AnimCellCulture_Guide.ashx
6. Morris CB. Planning and design of a cell and tissue culture laboratory.Safety in Cell and Tissue Culture.Springer Netherlands; 1998:87-101.doi:10.1007/978-94-011-4916-7_5
7. Herman P, Pauwels K. Biosafety Recommendations on the Handling of Animal Cell Cultures.Springer; 2015:689-716.doi:10.1007/978-3-319-10320-4_22
8. Warner DR, Sakai D, Sandell LL. Mammalian Cell Culture.Curr Protoc Essent Lab Tech.2015;10(1):4.3.1-4.3.33.doi:10.1002/9780470089941.et0403s10
9. Stacey G, MacDonald C. Immortalisation of Primary Cells.Cell Culture Methods for In Vitro Toxicology.Springer Netherlands; 2001:27-42.doi:10.1007/978-94-017-0996-5_3
10. Merten OW. Advances in cell culture: Anchorage dependence.Philos Trans R Soc B Biol Sci.2015;370(1661):20140040.doi:10.1098/rstb.2014.0040
11. Oyeleye OO, Ogundeji ST, Ola SI, Omitogun OG. Basics of animal cell culture: Foundation for modern science.Biotechnol Mol Biol Rev.2016;11(2):6-16.doi:10.5897/bmbr2016.0261
12. Marx V. Cell-line authentication demystified.Nat Methods.2014;11(5):483-488.doi:10.1038/nmeth.2932
13. Yao T, Asayama Y. Animal-cell culture media: History, characteristics, and current issues.Reprod Med Biol.2017;16(2):99-117.doi:10.1002/rmb2.12024
14. Price PJ. Best practices for media selection for mammalian cells.Vitr Cell Dev Biol - Anim.2017;53(8):673-681.doi:10.1007/s11626-017-0186-6
15. Coté RJ. Aseptic Technique for Cell Culture.Curr Protoc Cell Biol.1998;00(1):1.3.1-1.3.10.doi:10.1002/0471143030.cb0103s00
16. Davis JM, Shade KL. Aseptic Techniques in Cell Culture.Encyclopedia of Industrial Biotechnology.John Wiley & Sons, Inc.; 2009:1-20.doi:10.1002/9780470054581.eib059
17. Patil R, Kale A, Mane D, Patil D. Isolation, culture and characterization of primary cell lines of human buccal mucosal fibroblasts: A combination of explant enzamytic technique.J Oral Maxillofac Pathol.2020;24(1):68-75.doi:10.4103/jomfp.JOMFP_282_19
18. Freshney RI. Primary Culture.Culture of Animal Cells.John Wiley & Sons, Inc.; 2005.doi:10.1002/0471747599.cac012
19. Young L, Sung J, Masters JR. Detection of mycoplasma in cell cultures.Nat Protoc.2010;5(5):929-934.doi:10.1038/nprot.2010.43
20. Chen TR. In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain.Exp Cell Res.1977;104(2):255-262.doi:10.1016/0014-4827(77)90089-1
21. Cadena-Herrera D, Esparza-De Lara JE, Ramírez-Ibañez ND, et al. Validation of three viable-cell counting methods: Manual, semi-automated, and automated.Biotechnol Reports.2015;7:9-16.doi:10.1016/j.btre.2015.04.004
22. Absher M. Hemocytometer Counting.Tissue Culture.Elsevier; 1973:395-397.doi:10.1016/b978-0-12-427150-0.50098-x
23. Kim TK, Eberwine JH. Mammalian cell transfection: The present and the future.Anal Bioanal Chem.2010;397(8):3173-3178.doi:10.1007/s00216-010-3821-6
24. Azzam T, Domb A. Current Developments in Gene Transfection Agents.Curr Drug Deliv.2005;1(2):165-193.doi:10.2174/1567201043479902
25. Kumar P, Nagarajan A, Uchil PD. Lipofection.Cold Spring Harb Protoc.2019;2019(3):184-187.doi: 10.1101 / pdb.top096248




