如何选择合适的免疫印迹Detection Method

Western blotting is a molecular biology technique centered on the size-based separation and detection of proteins. It consists of sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) followed by the transfer of proteins onto a membrane (nitrocellulose or polyvinylidene difluoride - PVDF) and subsequent detection of proteins with primary and secondary antibodies. A detailed western blotting protocol can be foundhere.
It is important to understand the advantages and disadvantages of different ways of detecting a protein of interest on the membrane. Here, we present the three main types of protein detection for western blot: 1) radioactive, 2) enzymatic, and 3) fluorescent in Figure 1.
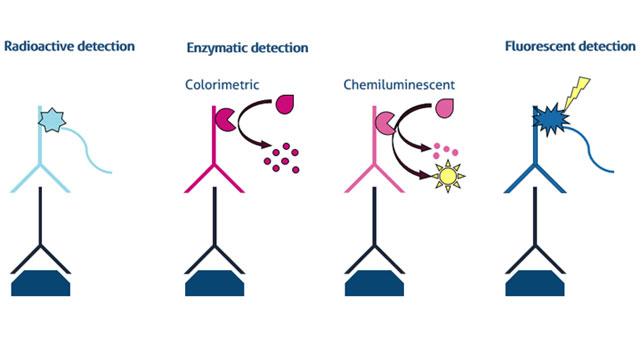
1.Radioactive detection
One of the first methods of primary antibody detection to be developed was the use of secondary antibodies labeled withradioisotopes(PMID: 388439). Iodine-125 was a popular choice with a relatively long half-life and emission of gamma rays consisting of low-energy photons that can be detected easily using X-ray films. Once a popular technique, its use nowadays is relatively rare.
Advantages: Radioactive detection offers good sensitivity and is very quantifiable.
Disadvantages:
•Quite laborious –secondary antibodies usually have to be labeled with radioisotopes by the end users.
•Quite expensive.
•Dangerous – requires specific radiation safety training, appropriate personal protective equipment (PPE), registry and waste disposal systems.
•Limited shelf-life of detection antibodies due to radioactive decay of the label.
Suggestions: Do not use, more contemporary methods are more effective – see below.
2.Enzymatic detection
最常见的一种方法,检测在韦斯tern blot is based on the use of a secondary antibody conjugated with an enzyme. The secondary antibody provides binding specificity towards the target protein recognized by the primary antibody, while the attached enzyme is used for detection. Following incubation of the membrane with the secondary antibody, solution containing specific substrate is applied to the membrane. The enzyme catalyzes conversion of a substrate, which is strictly localized to sites on the membrane where the secondary antibody bound to. The reaction results in formation of an insoluble colored precipitate on the membrane or release of photons that can be detected and quantified. This is the basis of colorimetric and chemiluminescent detections, respectively.
a.Colorimetric
Two of the most commonly used enzymes in colorimetric detection are alkaline phosphatase (AP) and horseradish peroxidase (HRP). After incubation of the western blot membrane with a secondary antibody conjugated with AP or HRP, the membrane is incubated with a solution containing a substrate for the enzyme. In the case of AP, the substrate is tetrazolium salts that are reduced to insoluble formazans. Chromogenic substrates for HRP include diaminobenzidine (DAB), tetramethylbenzidine (TMB), and azino-ethylbenzothiazoline-sulfonic acid (ABTS) salts. Chromogenic products of the enzymatic reaction on the western blot membrane are visible to the eye and mostly stable for a long time. The amount of precipitate can be quantified and corresponds to the amount of analyzed protein.
Advantages:
•Inexpensive and fast.
•The generated colored products are stable (hours to days), thus enabling imaging and quantification even weeks after blotting.
Disadvantages:
•Difficult to quantify – the user defines the duration of the precipitation reaction, which in most cases is irreversible. Limited linear range of the assay.
•Sensitivity – usually requires the presence of at least nanograms of analyzed protein to create visible bands, which is inferior to chemiluminescent and fluorescent detection methods.
Suggestions: Use for antibody screening and quick assessment.
b.Chemiluminescent
HRP and AP enzymes can also be used in chemiluminescent detection with appropriate substrates. HRP is more commonly used compared to AP due to a wider array of substrates and increased sensitivity achieved by using luminol- or acridan-based compounds. Similar to colorimetric detection, the western blot membrane is incubated with the secondary antibody conjugated with an enzyme and then a solution containing appropriate substrate is applied on the membrane. The intermediate products of the reaction produce low-energy photons that can be detected by placing the membrane against an X-ray film or gathered digitally by charge-coupled device (CCD) cameras. Unlike colorimetric detection, products are not insoluble and do not form visible precipitates on the membrane. The reaction is transient and requires detection within the first few minutes to hours. Commercially available HRP substrates offer great sensitivity.
Advantages:
•Sensitivity – femtograms of analyzed protein can be detected and quantified with the use of specific HRP substrates.
•Quantification – the use of digital cameras allows identification of overexposed membranes in order to ensure measurement within the linear range of detection.
Disadvantages:
•Timing of detection – light is emitted only during the conversion of a substrate into a product - detection needs to be performed immediately after addition of the substrate.
•Lack of multiplexing – if two examined proteins are similar in size, the simultaneous detection of both proteins is impossible with chemiluminescent detection. Consecutive detection is required – the first detection antibody needs to be stripped from the membrane prior to incubation with the antibody against the second target protein. Membrane stripping requires additional time and controls to ensure there is no protein loss from the membrane.
Suggestions: Ideal for quantification and when high sensitivity is needed due to low levels of analyzed protein in samples.
3.Fluorescence detection
In this method, the detection antibodies are not conjugated with enzymes but with fluorophores. Fluorophores are excited by light of a specific energy, which leads to the emission of light at a longer wavelength (e.g.; 680 nm for excitation and 694 nm for emission). The western blot membrane after incubation with secondary antibodies are imaged with a device equipped with an avalanche photodiode (APD) or CCD camera that detects the emitted light. Unlike enzymatic detection, no substrates are needed to enable detection. Used fluorophores are usually at the far-right end of the light spectrum (red and infrared) to minimize autofluorescence. Detection requires the use of dedicated equipment capable of illuminating membranes with light at a chosen spectrum. Sources of light are predominantly LEDs or lasers. Filter sets allow the specific detection of fluorophores and minimize background. The use of more than one fluorophore with non-overlapping emission spectra permits the simultaneous detection of more than one detection antibody with no need to wash and re-probe the membranes.
Advantages:
•Multiplexing – allows the simultaneous detection of two proteins of very similar molecular weight, when the detection antibody pair is conjugated with different fluorophores. This is particularly useful when comparing the abundance of phosphorylated protein to total protein levels.
•Stability – after incubation with detection antibodies, membranes can be stored for months at room temperature and signals detected at a convenient time.
•Simple to use – no additional steps are needed after incubation of the membrane with secondary antibodies prior to detection.
Disadvantages:
•Requires dedicated equipment for detection and image acquisition. Need to use special PVDF membranes due to the natural autofluorescence of standard PVDF membranes.
•Sensitivity – lower than chemiluminescent detection but with a wide linear range.
Suggestions: Ideal for quantification and multiplexing.
All of the detection methods presented offer advantages but also have limitations. It is important to optimize western blot protocol and experimental conditions in order to achieve the best results. Great results require a good signal-to-noise ratio, which can often be readily achieved by minimizing the background or improving weak/no signal, non-specific bands.




